PARKINSON's DISEASE
Home » Solutions / Drug Efficacy Testing / Movement Disorders / Parkinson's Disease
Side-effects and Tachyphylaxis: Room for Improvement in the Standard of Care
The prevalence of Parkinson’s Disease (PD) has doubled in the past 25 years*. This neurodegenerative disorder affects 0.3% of the world’s population and increases with age (1% in people over 60 and 4% in those over 80). It is characterized by degeneration of the dopaminergic neurons of the substantia nigra and aggregation of alpha synuclein and Lewy bodies, resulting in tremors, akinesia and bradykinesia.
To date, most effective treatments have significant side-effects, like Levo-dopa-Induced Dyskinesia (LID). These are caused directly by the mechanisms of action of the compounds, but unfortunately, we lack alternatives. Challenges such as the intricate molecular mechanisms combined with the selective permeability of the blood-brain barrier complicate the search for effective drugs. As symptoms become resistant in advanced stages, the pharmaceutical industry is striving to produce personalized approaches and reliable biomarkers. The journey from lab to market is long and costly, demanding innovative strategies and collaborative efforts to unlock new therapeutic frontiers.
BetaPark EEG Biomarker and 6-OHDA Rat Model: SynapCell's Preclinical Alternative to Boost Parkinson's Drug Discovery
SynapCell has developed a full spectrum of translational EEG services to address Parkinson’s Disease using validated biomarkers of the disease at different stages. From the symptomatic phase to LID, we can offer an evaluation of your compound from A-to-Z.
Our in vivo approach is unique, combining translational and predictive EEG Biomarkers combined with reliable and specific models. Our freely moving 6-OHDA rat model is clinically-relevant, non-behavior based, robust and objective. It is associated with BetaPark, the best translational EEG biomarker for Parkinson’s disease. BetaPark is progressively expressed in both PD patients and animal models (6-OHDA rat, MPTP, NHP MPTP…), allowing predictive and high-quality assessment of your drug candidates. The parallel with the clinic lies in the pharmacology and the EEG-based deficits found in both humans and 6-OHDA rats.
A Reference Model for Parkinson's Disease: 6-OHDA Rat
The model displays the EEG biomarker BetaPark, an abnormal beta oscillation in the motor cortex, which is also observed in patients and in other animal models of PD.

BetaPark, a Translational & pharmacosensitive EEG Biomarkers for Parkinson's Disease
The reason for the success of the 6-OHDA model lies in its EEG biomarker – an abnormal oscillation on the EEG spectrum, centered around the Beta frequency (12-30 Hz) – known as BetaPark.
BetaPark is sensitive to dopaminergic agonists, and is therefore also validated by the reference treatment. It represents an important tool in the development of PD drug candidates.

One Model with Two Applications
Key Features
of the 6-OHDA Model
The 6-OHDA model is considered a gold standard for drug discovery in Parkinson’s disease:
- Selectively targets dopaminergic neurons, mimicking the disease’s pathophysiology
- Reproduces motor deficits similar to Parkinson’s symptoms
- Allows study of disease symptoms
- Produces reproducible lesions
- Compatible with long-term evaluation of drug effects
- Can be combined with various assessment techniques
This model is a reliable preclinical research platform on which to assess potential Parkinson’s treatments.
The 6-OHDA rat model displays the same disorders as human patients with Parkinson’s disease over time, making it the ideal model to test drug efficacy for PD.
The first lesion appears quite rapidly (4-5 weeks) to generate the first EEG biomarker (BetaPark) but the model can then remain stable for several weeks, or months. This allows for longitudinal studies, to assess long-term chronic treatment or to allow crossover protocols with several conditions (up to 10).
SynapCell’s 6-OHDA rat model shows a translational and relevant pharmacology. Indeed, in this model, dopaminergic agonists such as ropinirole, L-DOPA, apomorphine, and SKF-38393 display dose-dependent effects on BetaPark reduction.
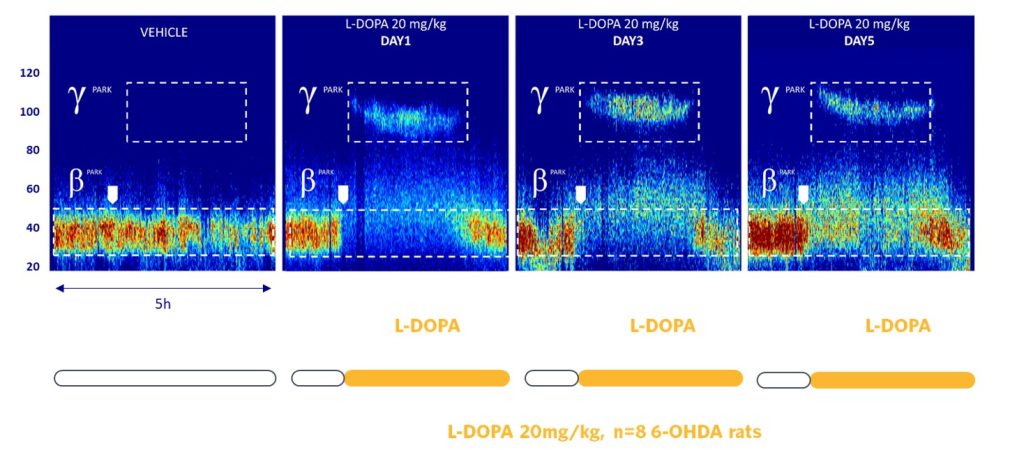
Similarly, the LID biomarker, GammaPark, is also pharmacosensitive to amantadine, the current standard of care.
Drug Discovery Assays with the 6-OHDA Model
Like many of the models in our portfolio, this model is stable over time and therefore compatible with crossover study designs.
These types of designs are well-suited for drug discovery as they enhance the statistical power of the data generated, limit the number of animals used, and allow a relatively fast turnaround for the first results.
In these settings, you can select the most promising candidate from a library of compounds, and/or perform another crossover to more precisely determine the effective dose of your lead asset.
Assess how a compound’s effects change with varying doses, and analyze the dose-response relationship, examining efficacy, safety, and biological effects across different concentrations to determine optimal dosing strategies.
Subtle effects can be highlighted thanks to the precision of EEG and the BetaPark biomarker, providing a very accurate dose-response curve.
Simulate the effects of short- and long-term levodopa (L-DOPA) treatment in Parkinson’s disease to study drug efficacy, side effects and disease progression over different time scales.
The acute effect of L-DOPA provides a very good positive control for the treatment of the symptoms while also offering a good control for undesirable side effects (GammaPark).
POSTER
Improving Drug Discovery in Parkinson's Disease Using Brain Oscillations as Translational Biomarkers
In this Poster, we investigate the use of aberrant cortical oscillations as translational biomarkers for Parkinson’s Disease Drug development. We evaluate the effect of acute (L-Dopa, Apomorphine) and chronic (L-Dopa) treatments on both EEG oscillations and Abnormal Involuntary Movements (AIMs) in the 6-OHDA model.
DOWNLOAD
POSTER

Powered by Cue®, SynapCell's Predictive In Vivo EEG Platform
SynapCell’s 6-OHDA rat model and its associated EEG biomarker (BetaPark) are processed on Cue®, our innovative translational in vivo EEG platform, which is designed to predict the in-human efficacy of your drug candidates during the preclinical step. Cue® is the result of decades of R&D, combining SynapCell’s know-how, expertise and scientific excellence in the fields of brain surgery and EEG signal recording, processing, and analysis.
Using Cue®, we transform preclinical data into actionable insights, offering end-to-end support for informed decision-making in CNS drug discovery.
THE SCIENCE CORNER
BetaPark, a Translational EEG Biomarker
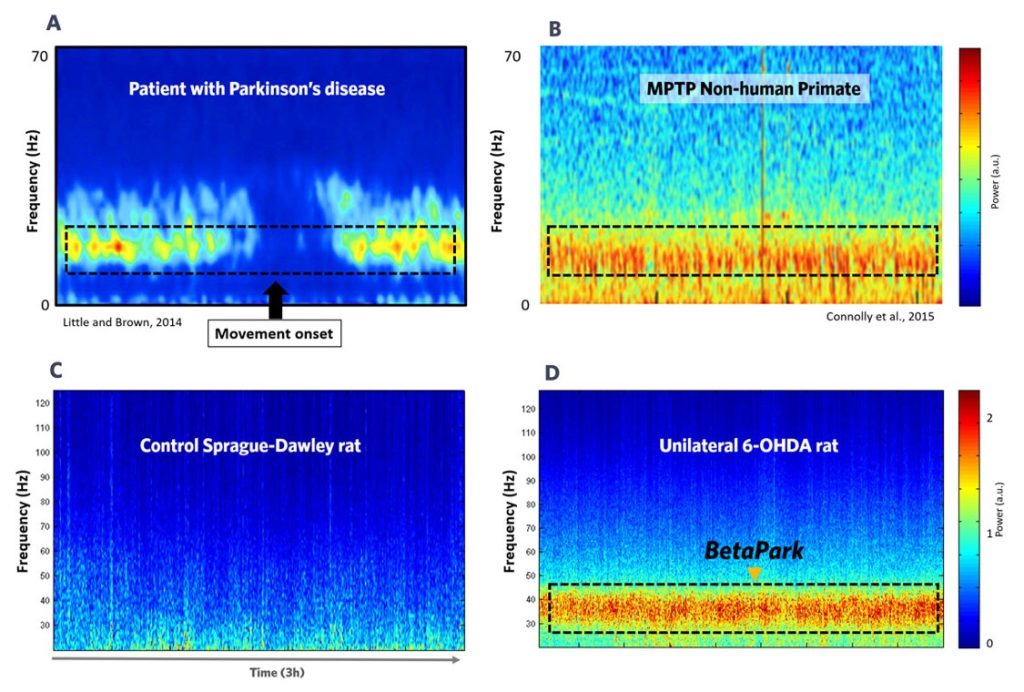
(A) In Parkinson’s Disease patients, an aberrant oscillation, around the beta band, is observed in the cortico-basal ganglia loop.
This aberrant oscillation is modulated by the initiation of voluntary movements, deep-brain stimulation and Dopaminergic drugs.
Adapted from Little and Brown 2014, Curr Biol. 2024 Sep 22; 24(18)
(B) Similar activity is observed in a non human primate model of PD, the MPTP model. In animals, a prominent and aberrant oscillation is also observed in the cortical-basal ganglia loop.
Adapted from Connolly et al. J neural Eng. 2015 Dec; 12(6):066012
(C) (D) Using the unilateral 6-OHDA lesioned rat model of PD, we found a prominent beta band (BetaPark, centered at 30Hz) in the motor cortex (D), which was absent from the EEG profile of control Sprague-Dawley rats (C).
The translational relevance of the EEG biomarker is greatly reinforced and supported by its presence in humans, NHP, and rodent models. The frequencies at which the abnormal oscillations are centered may vary slightly across species, but they are all correlated with movement and sensitive to the same drugs, as shown below.
Reference Pharmacology
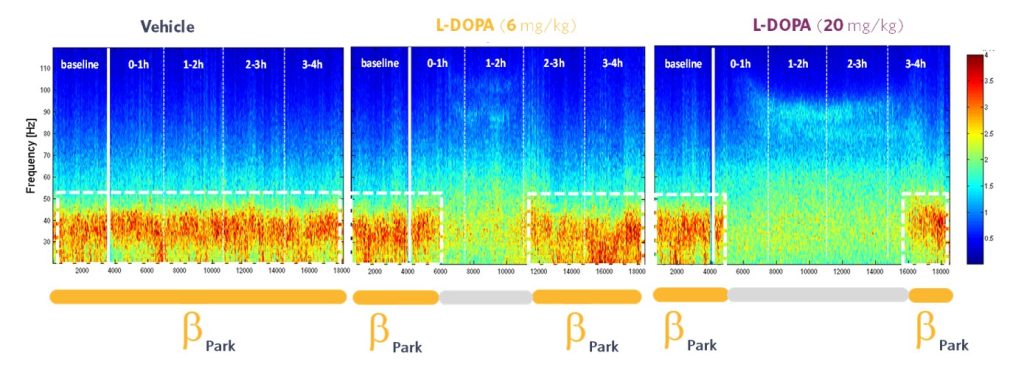
Diving into the details of the BetaPark EEG biomarker, the time-frequency maps above show the power of the classical frequency bands (1-140 Hz) as a function of time. Abnormally increased power is observed, centered around 30 Hz, and without pharmacological action, this increase is stable over time.
After administration of L-DOPA, the power observed at this frequency decreases. The effect lasts for about an hour and is dose dependent, not only in terms of the amplitude of the effect (the higher the dose, the greater the reduction in beta power), but also in terms of the duration of the effect. Indeed, as shown on the time-frequency maps, increasing the dose of L-DOPA induces a longer-lived effect.
GammaPark, a Dyskinesia-related EEG Biomarker
Let's Talk About Your Research Project!
More than a CRO, a team of collaborators – we are your dream neuroscience team specialized in preclinical EEG! We don’t just produce data, we are your partners from conceptualization to conclusion. We translate raw EEG data into meaningful, clinically-relevant endpoints, delivering clear insights to allow data-based decision-making. Choose SynapCell, a leading preclinical CNS-specialized CRO for cutting-edge EEG expertise combined with an irresistible touch of fun.
News & Events
PRESS RELEASE
SynapCell and the University of Utah Celebrate the 10-year Anniversary of their Collaboration on Anti-Seizure Medications.
NEW!
AMYGDALA KINDLING MODEL
Choose our Amygdala Kindling model to test compounds targeting focal-to-bilateral tonic-clonic seizures. Choosing the right model for the appropriate type of epilepsy seizures is key to the effective discovery of ASMs.
NEW!
SLEEP & VIGILANCE STATES
Discover SynapCell’s new preclinical EEG capabilities for sleep and vigilance states, and gain additional insights to characterize compound effects.