Focal to secondarily generalized Epilepsy
Home » Solutions / Drug Efficacy Testing / Epileptic Disorders » Focal to Secondarily Generalized Epilepsy
The Amygdala Kindling Rat: The Model of Choice for Testing Compounds Targeting Focal-to-Bilateral Tonic-Clonic Seizures
Epilepsy is the fourth most common human disorder worldwide according to the Epilepsy Foundation. However, there are several types of Epilepsies and seizures that differ not only in terms of EEG signals but also more fundamentally, in their physiopathology. The involvement of different structures means that, at the preclinical level, we need to tackle each type of Epilepsy with an appropriate, specific strategy.
Therefore, no single model is suitable for all Epilepsy types. This led us to develop the Amygdala Kindling rat model. Our goal was to offer a distinct model that complements SynapCell’s existing portfolio, which already includes models for Focal and Absence Epilepsy. With this new model, we expect to provide a powerful and robust tool to test compounds targeting refractory or difficult-to-treat forms of Epilepsy.

Combining EEG Biomarkers and Behavioral Endpoints at the Preclinical Stage for Greater Accuracy
While SynapCell’s core expertise lies in electrophysiological recording and analysis, we have added a behavioral endpoint for this particular model. We believe that combining the After Discharge Duration (ADD) EEG biomarker, which address focal and secondarily generalized seizures, with the well-established Racine scale, used to score convulsive seizures, provides a more comprehensive assessment.
This dual approach, integrating both electrophysiological and behavioral endpoints, is crucial for this model and offers a significant translational advantage. The combination of these two assessment methods provides a more robust and relevant preclinical evaluation of potential antiseizure medications targeting difficult-to-treat Epilepsies.
A Highly Predictive Model
The pharmacology tested to validate the model nicely reproduces what has been shown to be effective in the clinic.
In addition, Ethosuximide, which is used to effectively treat absence Epilepsy, has no effect on the model, as expected from the literature. For more information, check out the poster below!

Testing Two Types of Seizures on One Model
The rat Amygdala Kindling model offers the possibility to test a compound on two distinct types of seizures.
The potential efficacy of compounds on the mechanisms leading to focal seizures – originating from a specific structure, the amygdala – or on the mechanisms behind generalized seizures – affecting the whole brain – can be tested.
A Standardized Procedure for a Reproducible Model
The Amygdala Kindling rat model is tricky to fully master. Various protocols have been published the literature and some (crucial) details may vary from one team to another.
At SynapCell, we wanted to be sure our model was reproducible. We therefore put all of our R&D efforts into developing a protocol that is robust, reproducible and provides coherent data over different cohorts and at any time of the year.
Key Features of the Rat Amygdala Kindling Model
With this rat model, in a single test, it is possible to assess a compound’s efficacy on both focal and convulsive seizures. This dual test capacity is unique to this model, as we master the stimulation and seizure generation.
This model can be used to test the effect, or lack thereof, of a compound on focal seizures. These seizures are unique as, in a first phase, they do not spread to the rest of the cortex. You can therefore learn more about your drug by analyzing its effect on this type of seizure, monitoring its action at the core of the epilepsy and its capacity to stop the spreading.
Unchecked focal seizures will eventually spread, translating into secondarily generalized seizures. To act on these secondarily generalized seizures, a different mechanism of action is required, and studies may provide additional information on the clinical potential of your compound. For example, some drugs may not be able to contain the focal seizures in the amygdaloid complex, but will have an effect on the spreading of the discharge to the rest of the brain.
Drug Discovery Assays with the Rat Amygdala Kindling Model
For crossover protocols, each animal can be its own control. This limits the number of animals required for each study. This setup provides an efficacious means to screen compounds.
Once a lead has been selected through a screening protocol, a second crossover can be imagined to fine-tune the dose of the efficacious compound identified. Dose-response protocols are a crucial step in preclinical drug development.
Once the kindling process is terminated, responsive animals will be enrolled in the crossover and/or the chronic treatment studies.
After responsiveness has been validated, the model remains stable thanks to SynapCell’s specific protocol. The stimulation will continue to induce similar responses and seizures for several weeks.
DOWNLOAD
POSTER
POSTER
Pharmacological Assessment of Anti-Seizure Medications in the Rat Amygdala Kindling Model
This study highlights translational and predictive aspects of the Amygdala Kindling rat model as an asset for characterizing new treatment entities with improved tolerability and efficacy.
The pharmacology includes a wide range of ASM, with positive and negative controls. Both readouts (EEG biomarkers and Racine scale scoring), were performed in house.

Powered by Cue®, SynapCell's Predictive In Vivo EEG Platform
SynapCell’s rat Amygdala Kindling model and its associated EEG biomarker (ADD) are processed on Cue®, our innovative translational in vivo EEG platform, which is designed to predict the in-human efficacy of your drug candidates during the preclinical step. Cue® is the result of decades of R&D, combining SynapCell’s know-how, expertise and scientific excellence in the fields of brain surgery and EEG signal recording, processing, and analysis.
Using Cue®, we transform preclinical data into actionable insights, offering end-to-end support for informed decision-making in CNS drug discovery.
THE SCIENCE CORNER
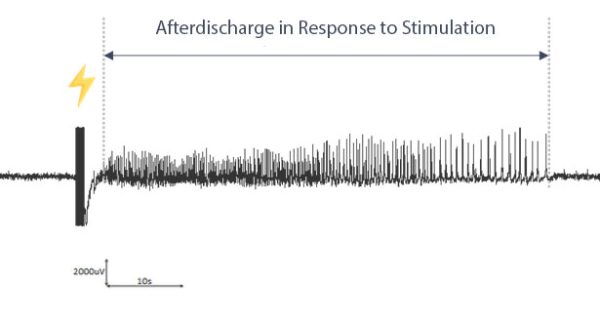
After Discharge Duration (ADD): One of the Most Relevant EEG Biomarker for this Model
ADD is one of the main endpoints we use in this model. It represents the total time over which the system (here, the amygdaloid complex) responds to the electrical stimulation.
In other words, ADD corresponds to a prolonged spike activity that follows a brief electrical stimulus. It is increased when the system is prone to seizures, after the full kindling process. The ADD can be reduced by the reference treatment (see poster above), and this reduction translates into an alleviation of the disease burden for patients. It is therefore one of the most relevant EEG biomarkers for this model.
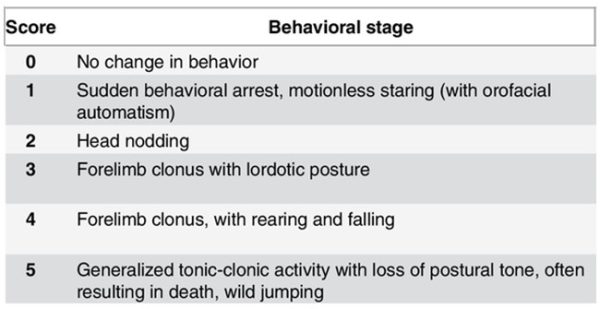
Racine Scale
This scale is extensively described in the literature, where it is used to classify and characterize the intensity of a behavioral seizure.
Although, like other behavioral endpoints, there may be some bias and subjectivity, the Racine scale represents an important parameter to combine with an objective method such as EEG. Each level of the scale is associated with a typical animal behavior, as shown in the adjacent table.
Let's Talk About Your Research Project!
More than a CRO, a team of collaborators – we are your dream neuroscience team specialized in preclinical EEG! We don’t just produce data, we are your partners from conceptualization to conclusion. We translate raw EEG data into meaningful, clinically-relevant endpoints, delivering clear insights to allow data-based decision-making. Choose SynapCell, a leading preclinical CNS-specialized CRO for cutting-edge EEG expertise combined with an irresistible touch of fun.
News & Events
PRESS RELEASE
SynapCell and the University of Utah Celebrate the 10-year Anniversary of their Collaboration on Anti-Seizure Medications.
NEW!
AMYGDALA KINDLING MODEL
Choose our Amygdala Kindling model to test compounds targeting focal-to-bilateral tonic-clonic seizures. Choosing the right model for the appropriate type of epilepsy seizures is key to the effective discovery of ASMs.
NEW!
SLEEP & VIGILANCE STATES
Discover SynapCell’s new preclinical EEG capabilities for sleep and vigilance states, and gain additional insights to characterize compound effects.