Focal Epilepsy
Home » Solutions / Drug Efficacy Testing / Epileptic Disorders » Focal Epilepsy
Overcoming MTLE: A Major Challenge for Drug Discovery in Epilepsy
Mesial Temporal Epilepsy (MTLE) – or Focal Epilepsy – affects over 10% of patients with Epilepsy and represents a major challenge, as most pharmacological treatments are ineffective against these types of seizures. Despite the availability of over 20 anti-seizure medications, important unmet medical needs remain, with drug-resistance being a problem for about one-third of patients with epilepsy.
There are several models of epilepsy that can be used to test novel compounds, but not all models are equal or appropriate to study effects on non-convulsive MTLE. For drug developers, using the right non-convulsive model that perfectly mimics human MTLE at the preclinical stage is key, allowing accurate assessment of the efficacy of drug candidates.
SynapCell's EEG Biomarker in the MTLE Mouse Model: A Powerful Combination to Assess Drug Efficacy for Focal Epilepsy
Our in vivo EEG Biomarker approach is what makes SynapCell unique. The models in our portfolio express translational EEG biomarkers that are identical to human EEG biomarkers. Our MTLE model and its related EEG biomarker (HPD – Hippocampal Paroxysmal Discharges) are a powerful combination, delivering smooth and relevant human-rodent translation, high reproducibility, and robust endpoints. This duo is particularly well-suited for the unbiased evaluation of how compounds affect Focal Epilepsy and can predict the potential impact of a molecule throughout the different clinical phases.
SynapCell's MTLE Model Perfectly Mimics Human MTLE
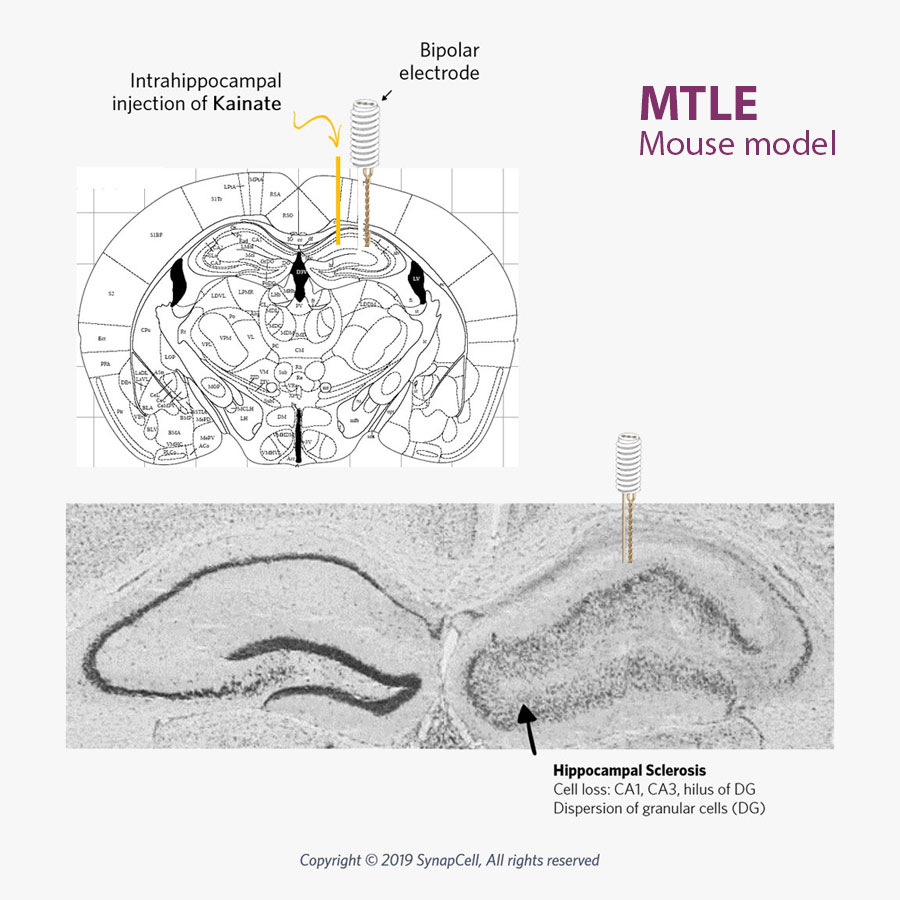
Our MTLE mouse model mirrors human MTLE, with the same histological, electro-physiological, and pharmacological features as human pharmaco-resistant and non-convulsive MTLE.
This model is therefore the most relevant predictive model to qualify Anti-Seizure Medications (ASMs).

A Predictive Approach with Translational EEG Biomarkers
SynapCell’s MTLE model uses advanced EEG biomarkers to monitor non-convulsive seizures, mirroring human epilepsy. This innovative approach, developed through extensive R&D, offers drug discoverers crucial insights when assessing molecule efficacy at the preclinical stage, to accelerate the development of epilepsy treatments.
SynapCell's MTLE Model is a Reference on the Market
Unique know-how in MTLE modeling makes SynapCell’s MTLE model the most robust translational model for Focal Epilepsy on the market today.
SynapCell partners with a world-renowned Epilepsy expert, approved by the NINDS ETSP Program.

“Drug-resistant Epilepsies remain a challenge to this day, and there is a need for translational models to empower preclinical drug discovery. SynapCell’s MTLE mouse model is clinically relevant as it closely replicates the physiopathological, electrophysiological, and pharmacological features of human temporal lobe epilepsy. Having this model available in the ADD Program is a great asset for the ETSP.”
Prof. Karen S. Wilcox, Director of the ADD Program
From Small Molecules to Gene Therapies, SynapCell’s Assessments Can Test a Wide Range of Medications
Key Features
of the MTLE Mouse Model
SynapCell’s MTLE model and its associated HPD EEG biormarker are recognized as one of the most robust, relevant and translational models for the evaluation of compounds for Focal Epilepsy. This model is used by world-renowned epilepsy experts and has been approved by the NINDS ETSP program.
SynapCell’s non-convulsive mouse model of MTLE mirrors human pharmaco-resistant MTLE with the same histological, electro-physiological, and pharmacological features. This model is recognized as one of the most robust and translational models for Focal Epilepsy available on the market today.
The MTLE mouse is a non-convulsive model of focal, pharmacoresistant epilepsies that perfectly mirrors human non-convulsive focal, pharmacoresistant MTLE Epilepsy.
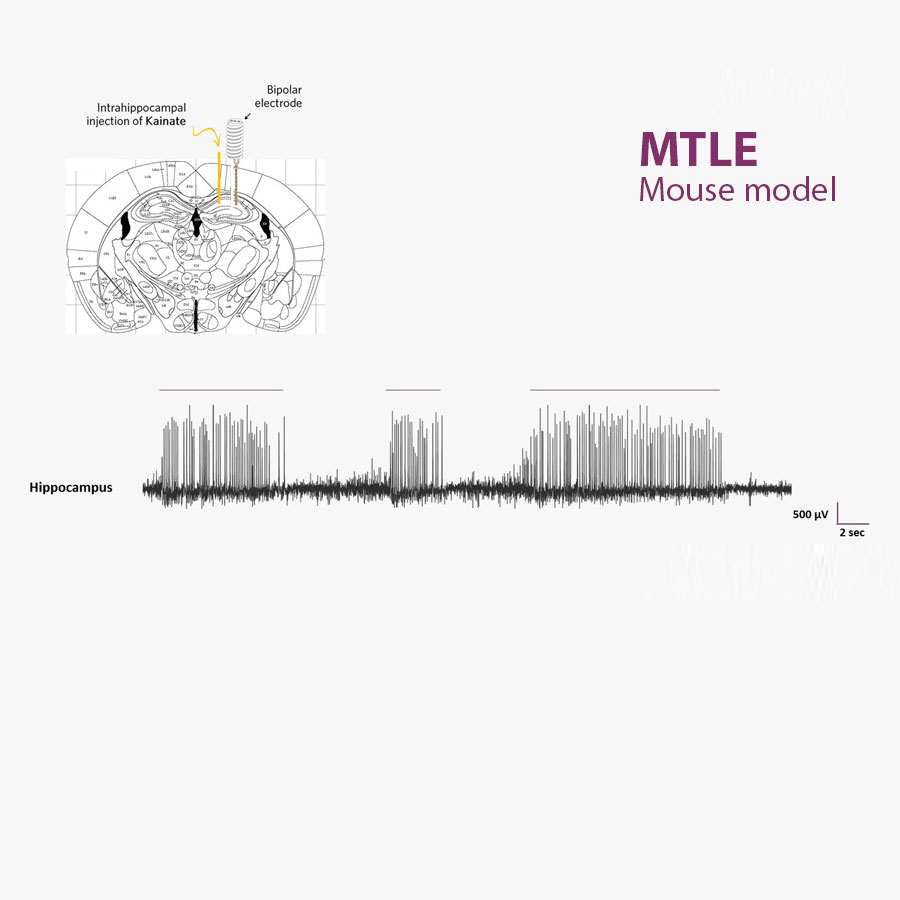
The MTLE mouse model is non-convulsive. Seizures are monitored within the brain using appropriate EEG methods and based on EEG biomarkers (HPD).
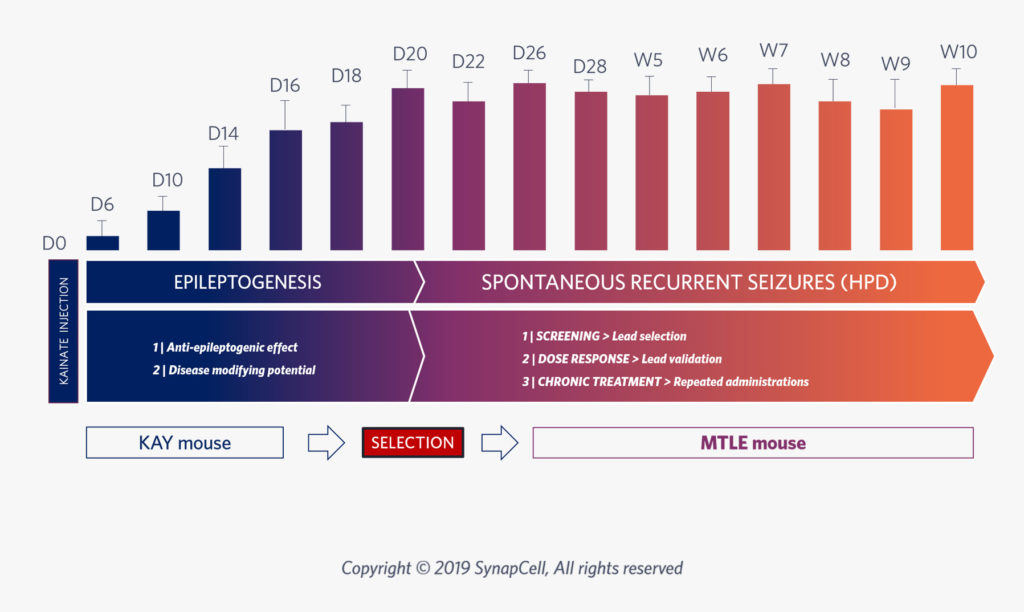
After 3 weeks of epileptogenesis, the MTLE mouse model shows the same epileptic features as human MTLE, with non-convulsive and drug-resistant seizures. It is thus the ideal model to test drug efficacy for Focal Epilepsy.
The MTLE mouse is a non-convulsive model of focal, pharmacoresistant epilepsies. Its EEG reveals spontaneous, recurrent Hippocampal Paroxysmal Discharges (HPD), which can be used as objective EEG biomarkers to reliably evaluate the effects of antiseizure medications (ASMs) in vivo.
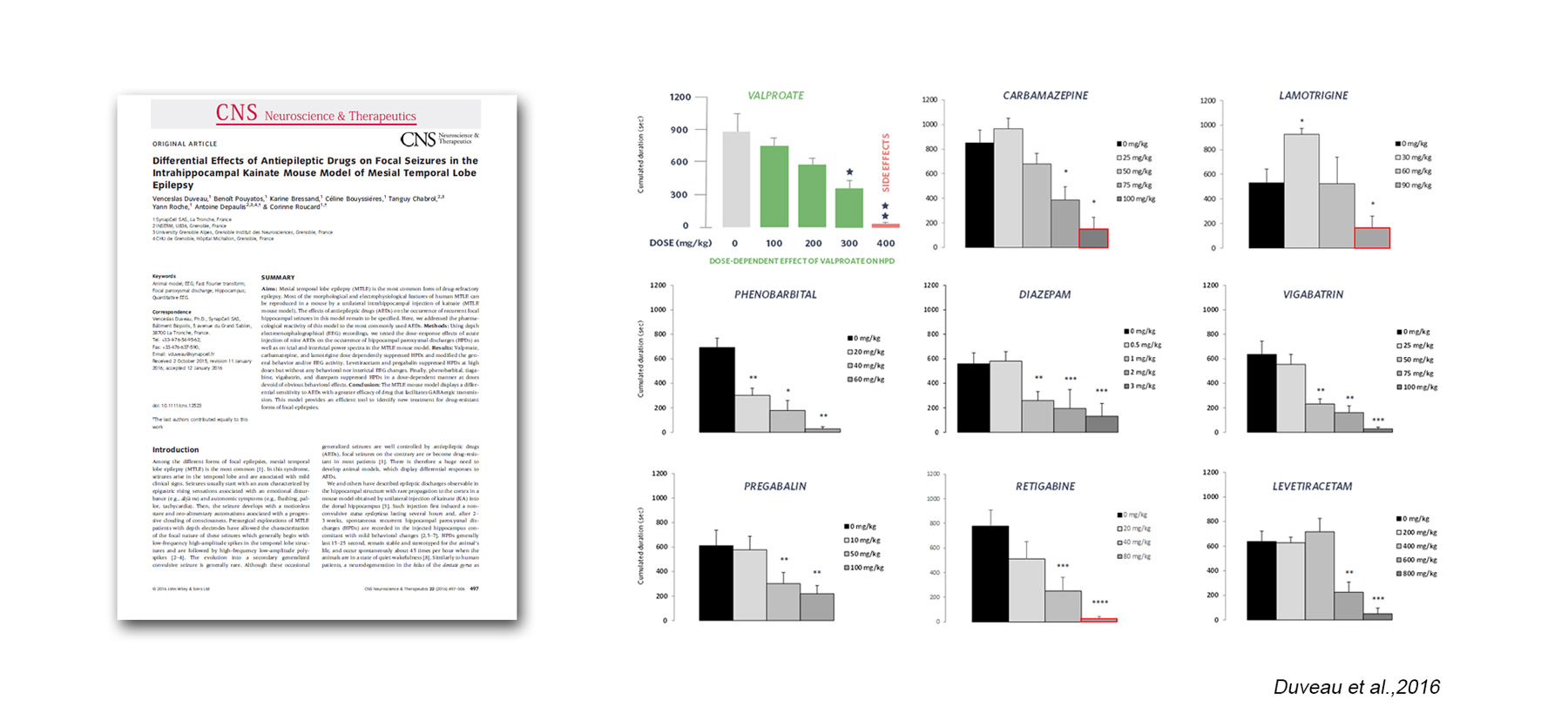
SynapCell’s MTLE mouse model is validated by pharmacological reference compounds:
Voltage-gated channel:
– valproate
– carbamazepine
– lamotrigine
– retigabine
Gabaergic system:
– diazepam
– phenobarbital
– vigabatrin
Other parameters:
– levetiracetam
– pregabalin
Drug Discovery Assays with the MTLE Mouse Model
Rapidly test a library of compounds to identify potential drug candidates.
Identify, optimize and test the efficacy and pharmacological properties of promising compounds to determine their potential as drug candidates.
Monitor the reactions of biological systems to varying drug concentrations. The resulting dose-response curve provides vital information on drug potency, efficacy, therapeutic range, and safety, guiding crucial decisions in drug development.
Epileptogenesis, whereby a healthy brain develops a tendency for recurrent seizures, is a key focus for developing new anti-seizure medications that aim to prevent, modify, or reverse the progression of epilepsy, rather than just treating symptoms.
Assess your compound’s potential to modify Epilepsy in the long-term.
Gene therapy for Focal Epilepsy involves introducing genetic material into specific brain regions to modify neuronal excitability, potentially reducing seizures in drug-resistant cases. This approach is gradually emerging in epilepsy treatment discovery.
The MTLE model associated with its EEG biomarker can be used in a large range of assays to test compounds for Epilepsy… but it can do more! This model is also relevant when evaluating the potentiating or synergistic effects of anti-seizure medications in combination with other drugs and can be used to identify the most effective drug associations.
An MTLE study (focal epilepsy) can also be combined with a GAERS study (generalized epilepsy) to derisk your compounds, identify seizure-aggravating effects and, more importantly, align your preclinical strategy with your clinical roadmap to validate your anti-seizure medication (ASM).
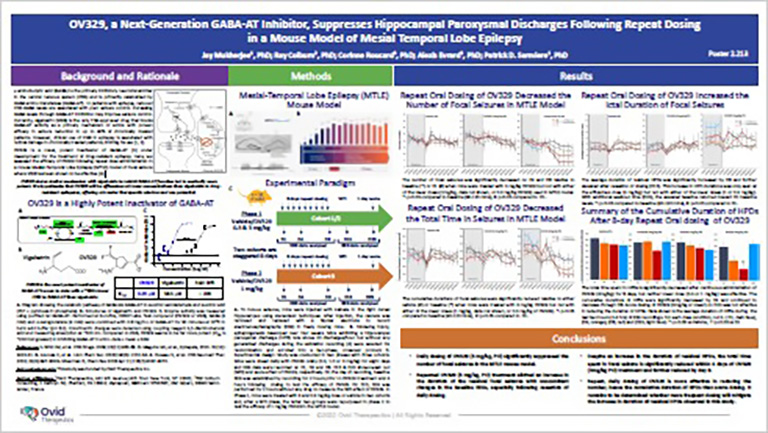
POSTER
The SynapCell MTLE Model, a Predictive Model of Focal Epilepsy for Drug Discovery
In this poster, discover a powerful preclinical tool to accelerate drug-resistant Epilepsy research. The MTLE model accurately mirrors human focal Epilepsy, reproducing drug sensitivities and crucial EEG biomarkers. Revolutionize MTLE treatment strategies with this invaluable resource, which gives you unparalleled insights and an advanced understanding of epilepsy, to promote the development of innovative therapies.
DOWNLOAD
POSTER

Powered by Cue®, SynapCell's Predictive In Vivo EEG Platform
SynapCell’s MTLE mouse model and its associated EEG biomarker (HPD) are processed on Cue®, our innovative translational in vivo EEG platform, which is designed to predict the in-human efficacy of your drug candidates during the preclinical step. Cue® is the result of decades of R&D, combining SynapCell’s know-how, expertise and scientific excellence in the fields of brain surgery and EEG signal recording, processing, and analysis.
Using Cue®, we transform preclinical data into actionable insights, offering end-to-end support for informed decision-making in CNS drug discovery.
THE SCIENCE CORNER
MTLE Hippocampal Sclerosis
Mesial Temporal Lobe Epilepsy involves seizures originating in the internal structures of the temporal lobe, in particular the hippocampus. Human MTLE epilepsy often results from sclerosis of the hippocampus. This hippocampal sclerosis is reproduced in SynapCell’s MTLE model.
HPD, SynapCell's EEG Biomarker for the MTLE Model
Hippocampal Paroxysmal Discharges (HPDs) are EEG biomarkers of the brain hyperexcitability observed in epilepsy. They are characterized by bursts of high-frequency spikes and sharp waves, and typically last for several seconds. HPDs originate in the hippocampus, particularly the dentate gyrus, subsequently propagating to other regions. They are considered non-convulsive focal seizures and can be used to study epilepsy and test new therapies. HPDs provide valuable insights into epileptogenesis and serve as a tool to evaluate potential antiepileptic treatments in preclinical research.
Compare your Compound to the Standard of Care and Demonstrate Superior Effects
The model-EEG biomarker duo (MTLE mouse – HPD) has been validated with a reference treatment and been used to demonstrate differential sensitivity to ASMs, with drugs that facilitate GABAergic transmission showing greater efficacy. This is what makes the model a powerful tool to identify new treatments for drug-resistant forms of focal epilepsies.
Joint Work with Ovid Therapeutics

Ovid Therapeutics’ OV329 is a novel, potent inactivator of GABA-AT under development for the treatment o fdrug-resistant epilepsy. Here, the efficacy of OV329 is assessed following repeat dose administration in SynapCell’s Mesial-Temporal Lobe Epilepsy (MTLE) mouse model of focal seizure where VGB has been shown to be effective.
Let's Talk About Your Research Project!
More than a CRO, a team of collaborators – we are your dream neuroscience team specialized in preclinical EEG! We don’t just produce data, we are your partners from conceptualization to conclusion. We translate raw EEG data into meaningful, clinically-relevant endpoints, delivering clear insights to allow data-based decision-making. Choose SynapCell, a leading preclinical CNS-specialized CRO for cutting-edge EEG expertise combined with an irresistible touch of fun.
News & Events
PRESS RELEASE
SynapCell and the University of Utah Celebrate the 10-year Anniversary of their Collaboration on Anti-Seizure Medications.
NEW!
AMYGDALA KINDLING MODEL
Choose our Amygdala Kindling model to test compounds targeting focal-to-bilateral tonic-clonic seizures. Choosing the right model for the appropriate type of epilepsy seizures is key to the effective discovery of ASMs.
NEW!
SLEEP & VIGILANCE STATES
Discover SynapCell’s new preclinical EEG capabilities for sleep and vigilance states, and gain additional insights to characterize compound effects.